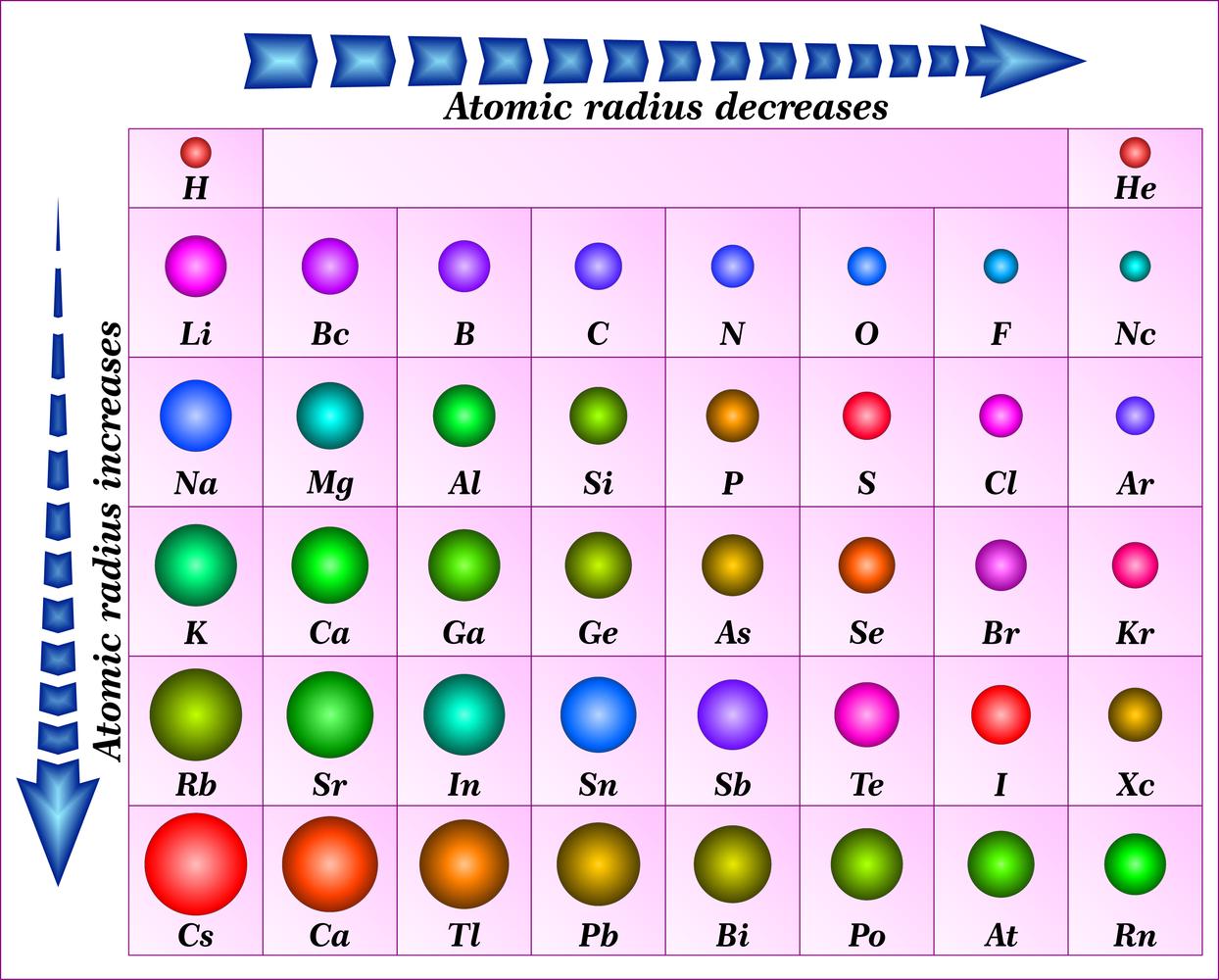
Atomic Radius Definition Britannica . An atom has no rigid. The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas. On the periodic table, atomic radius generally decreases as you move from left to right across a period. Atomic radius, half the distance between the nuclei of identical neighbouring atoms in the solid form of an element. The model described the atom as a. How atomic radius is defined, and trends across a period and down a group. A convenient unit of length for measuring atomic sizes is the angstrom (å), defined as 10 −10 metre. The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the.
from 88guru.com
The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the. How atomic radius is defined, and trends across a period and down a group. A convenient unit of length for measuring atomic sizes is the angstrom (å), defined as 10 −10 metre. The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas. On the periodic table, atomic radius generally decreases as you move from left to right across a period. Atomic radius, half the distance between the nuclei of identical neighbouring atoms in the solid form of an element. An atom has no rigid. The model described the atom as a.
Atomic RadiusAn Overview 88Guru
Atomic Radius Definition Britannica The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas. The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas. A convenient unit of length for measuring atomic sizes is the angstrom (å), defined as 10 −10 metre. The model described the atom as a. The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the. How atomic radius is defined, and trends across a period and down a group. On the periodic table, atomic radius generally decreases as you move from left to right across a period. Atomic radius, half the distance between the nuclei of identical neighbouring atoms in the solid form of an element. An atom has no rigid.
From neetlab.com
Atomic Radius Periodic Table NEET Lab Atomic Radius Definition Britannica The model described the atom as a. An atom has no rigid. The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas. How atomic radius is defined, and trends across a period and down a group. A convenient unit of length for measuring atomic sizes is the. Atomic Radius Definition Britannica.
From animalia-life.club
Atomic Radius Diagram Atomic Radius Definition Britannica Atomic radius, half the distance between the nuclei of identical neighbouring atoms in the solid form of an element. The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas. A convenient unit of length for measuring atomic sizes is the angstrom (å), defined as 10 −10 metre.. Atomic Radius Definition Britannica.
From www.shutterstock.com
713 Atomic Radius Images, Stock Photos, 3D objects, & Vectors Atomic Radius Definition Britannica An atom has no rigid. The model described the atom as a. The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the. Atomic radius, half the distance between the nuclei of identical neighbouring atoms in the solid form of an element. On the. Atomic Radius Definition Britannica.
From www.youtube.com
periodic properties/atomic radius/atomic radius definition/limitations Atomic Radius Definition Britannica On the periodic table, atomic radius generally decreases as you move from left to right across a period. Atomic radius, half the distance between the nuclei of identical neighbouring atoms in the solid form of an element. The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from. Atomic Radius Definition Britannica.
From ezchem.com
Atomic Radius Trend EZchem Atomic Radius Definition Britannica The model described the atom as a. An atom has no rigid. How atomic radius is defined, and trends across a period and down a group. On the periodic table, atomic radius generally decreases as you move from left to right across a period. The atomic radius of a chemical element is a measure of the size of its atom,. Atomic Radius Definition Britannica.
From periodicitychem.weebly.com
Atomic Radius Periodicity Project Atomic Radius Definition Britannica Atomic radius, half the distance between the nuclei of identical neighbouring atoms in the solid form of an element. On the periodic table, atomic radius generally decreases as you move from left to right across a period. The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from. Atomic Radius Definition Britannica.
From study.com
What is Atomic Radius? Atomic Radius Examples & Periodic Trend Atomic Radius Definition Britannica How atomic radius is defined, and trends across a period and down a group. The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas. The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from. Atomic Radius Definition Britannica.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID1951146 Atomic Radius Definition Britannica The model described the atom as a. Atomic radius, half the distance between the nuclei of identical neighbouring atoms in the solid form of an element. On the periodic table, atomic radius generally decreases as you move from left to right across a period. How atomic radius is defined, and trends across a period and down a group. A convenient. Atomic Radius Definition Britannica.
From www.slideserve.com
PPT Anatomy of the Periodic Table PowerPoint Presentation, free Atomic Radius Definition Britannica An atom has no rigid. The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas. How atomic radius is defined, and trends across a period and down a group. The atomic radius of a chemical element is a measure of the size of its atom, usually the. Atomic Radius Definition Britannica.
From mungfali.com
Types Of Atomic Radius Atomic Radius Definition Britannica Atomic radius, half the distance between the nuclei of identical neighbouring atoms in the solid form of an element. A convenient unit of length for measuring atomic sizes is the angstrom (å), defined as 10 −10 metre. The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas.. Atomic Radius Definition Britannica.
From general.chemistrysteps.com
Atomic Radius Chemistry Steps Atomic Radius Definition Britannica Atomic radius, half the distance between the nuclei of identical neighbouring atoms in the solid form of an element. The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the. The model described the atom as a. How atomic radius is defined, and trends. Atomic Radius Definition Britannica.
From www.chemistrystudent.com
Chemistry Student Alevel Chemistry guides, notes and free revision Atomic Radius Definition Britannica A convenient unit of length for measuring atomic sizes is the angstrom (å), defined as 10 −10 metre. How atomic radius is defined, and trends across a period and down a group. The model described the atom as a. On the periodic table, atomic radius generally decreases as you move from left to right across a period. Atomic radius, half. Atomic Radius Definition Britannica.
From dfwlokasin.weebly.com
Atomic radius definition dfwlokasin Atomic Radius Definition Britannica The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the. How atomic radius is defined, and trends across a period and down a group. The model described the atom as a. A convenient unit of length for measuring atomic sizes is the angstrom. Atomic Radius Definition Britannica.
From slideplayer.com
Trends & the Periodic Table ppt download Atomic Radius Definition Britannica An atom has no rigid. The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the. The covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas. How atomic radius is defined,. Atomic Radius Definition Britannica.
From slideplayer.com
The Periodic Table III. Periodic Trends. ppt download Atomic Radius Definition Britannica On the periodic table, atomic radius generally decreases as you move from left to right across a period. Atomic radius, half the distance between the nuclei of identical neighbouring atoms in the solid form of an element. A convenient unit of length for measuring atomic sizes is the angstrom (å), defined as 10 −10 metre. The atomic radius of a. Atomic Radius Definition Britannica.
From periodictableguide.com
Get the Periodic table with Atomic radius values (Img+Chart) Atomic Radius Definition Britannica On the periodic table, atomic radius generally decreases as you move from left to right across a period. A convenient unit of length for measuring atomic sizes is the angstrom (å), defined as 10 −10 metre. Atomic radius, half the distance between the nuclei of identical neighbouring atoms in the solid form of an element. The covalent atomic radius (r. Atomic Radius Definition Britannica.
From www.learnatnoon.com
Atomic size and atomic radius explained Noon Academy Atomic Radius Definition Britannica Atomic radius, half the distance between the nuclei of identical neighbouring atoms in the solid form of an element. The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the. On the periodic table, atomic radius generally decreases as you move from left to. Atomic Radius Definition Britannica.
From animalia-life.club
Atomic Radius Diagram Atomic Radius Definition Britannica Atomic radius, half the distance between the nuclei of identical neighbouring atoms in the solid form of an element. On the periodic table, atomic radius generally decreases as you move from left to right across a period. The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from. Atomic Radius Definition Britannica.